Interventional Research
Interventional research essentially centers on evaluating the influence of a given intervention on various outcomes experienced by the individuals being studied. Examples of interventions include surgical procedures, new drugs, and prescribed courses of action to change a lifestyle (such as a vigorous exercise program) (AFMC 2012). An educational program is another example of an intervention.
Interventional research is characterized by the manipulation of the main intervention being investigated and the overall study environment. Within interventional research, researchers control how much of an intervention is received by study subjects, how often subjects are observed, and what variables of interest are measured when subjects are being observed.
Through manipulating the experience of subjects within the study environment, researchers can exert a greater level of control over potentially conflicting influences that may muddle their assessment of the true measure of the intervention’s effect on the study population. By exercising this greater level of control, researchers can have a greater level of confidence in the validity of the results of interventional studies than they could have using observational study designs that investigate influences on individuals in “real-life” circumstances.
Types of Interventional Studies
Interventional studies may be categorized into two broad types:
Preventive Studies
In the realm of social and behavioral research, preventive studies may be conducted to assess the effectiveness of various types of public health initiatives in reducing the risk of undesirable or harmful outcomes. For example, researchers might be interested in conducting a study to assess whether an education program had an influence on sexual behavior that could lead to a reduction in the risk of HIV transmission.
Researchers might also be interested in examining whether a particular intervention of interest (such as lifestyle changes or a drug) may prevent the development of a disease or particular health outcome. If researchers were conducting a trial of this type, they would want the subjects to be healthy individuals, but considered at risk of developing the particular condition or disease of interest. For example, diabetes prevention interventions might be delivered to people who are overweight or who have specific genetic profiles.
Therapeutic Studies
Researchers often carry out interventional studies to test a therapeutic regimen on individuals who are affected by some type of illness. These therapeutic studies typically test an experimental agent’s ability to treat the symptoms of an illness and limit the morbidity, mortality, or disability that may result from a particular disease.
Types of Interventional Study Designs
We will discuss various 6 types of interventional study designs, including:

1.Placebo-Controlled Design
The hallmark of experimental study designs is the comparison of two or more groups, with study subjects assigned to the study groups randomly. When choosing the type of comparison group (sometimes referred to as the control group) for a study, researchers will have to consider two broad areas: How does the study design and use of control groups affect the research's scientific credibility?; and Is the study ethical? Does the study protect the rights, safety, and welfare of the research subjects?
What is a placebo?
Placebo is an inert or innocuous substance used especially in controlled experiments testing the efficacy of another substance (such as a drug) (Merriam-Webster 2018). Placebos have also been referred to as “dummy pills” or “dummy treatments” (Bartz 2011) because they are not active treatments. A placebo is made to appear as identical as possible to the experimental intervention (test treatment) so study subjects may not readily discern to what group they have been randomized. Fake or sham surgeries are also used to employ the use of the placebo design. Sham surgery has been used in studies of subjects with Parkinson’s disease (Cherkasova and Stoessl 2014).
Use of Placebos
Placebos are used as a control for other extraneous, confounding factors that might affect the outcome researchers have chosen to study. When researchers conduct an interventional study, the two study groups should be as similar as possible. If the only difference between the groups is that one group receives the intervention and the other group does not, then researchers can have more confidence in reporting that any differences observed in the outcome of interest (during or at the end of the trial) were due specifically to the intervention. If researchers only administered an intervention to one group of study subjects, without comparison to a group that did not receive the intervention, they would not be able to know for certain whether an observed outcome was due to the intervention they administered. For example, people may have improved simply due to the passage of time. In this case, the use of a placebo can rule out the passage of time as an explanation for the findings.
Placebo Effect
Placebo conditions may create additional problems when researchers try to interpret the results, through a mechanism known as the placebo effect. The placebo effect is a favorable response to some type of intervention (for example, a pill, procedure, or counseling session) that does not have a direct physiological effect (Harvard Medical School 2012). The placebo effect seems to be especially evident in conditions that are defined primarily by symptoms (such as depression, migraine, and back pain) (Harvard Medical School 2012).
Hawthorne Effect
In addition to a placebo intervention having an effect on the outcome of interest, researchers may also find that individuals exhibit effects simply due to the fact that they are being observed. This discovery traces back to several behavioral experiments that were carried out at the Western Electric Company’s telephone plant in Hawthorne, Chicago, in the 1920s and early 1930s (Singleton and Straits 2005). The studies were conducted to assess whether changes in working conditions could have an effect on workers’ behaviors as they related to productivity. Various conditions within the work environment were changed (such as the timing and length of work breaks, lighting, and methods of payment). Initially, the researchers found that productivity increased after these conditions were improved. However, when the researchers returned these conditions back to their original states, something surprising happened: productivity continued to increase.
The researchers concluded that the workers were not simply responding to the changed working conditions. They reasoned that the workers were also responding to the special attention that was being given to them. This has come to be known as the Hawthorne effect. The Hawthorne effect holds that the experiment itself can have an effect on the subjects. The simple fact that study subjects are aware they are under study could have an effect on the outcome researchers are examining. Many subsequent studies have supported the conclusion that people behave differently when being observed in a research study (Barnes 2010).
Placebo and Hawthorne Effect
These phenomena are relevant to the field of testing new drugs. For example, consider subjects who are in a study testing a new drug to treat depression. If the subjects knew that they were possibly getting a drug believed to help their mental state, this could cause a change in their depression (that is, placebo effect). In addition, the extra attention paid to subjects might actually improve their depression (that is, the Hawthorne effect).
Therefore, it is possible that, if researchers have two groups and give a new drug to one and placebo to the other, both groups could get better. If the experiment itself has an effect, both groups should be affected. However, if researchers have a drug that works, they should see even greater improvements in the group receiving the real drug. So, although the Hawthorne effect may cause researchers to overestimate the magnitude of the intervention’s effect, it should not affect their assessment of the difference between the intervention and control groups (McCarney et al. 2007). The Hawthorne effect should be observed in both groups.
Natural Course of a Condition or Disease
The natural course of a condition or disease could also have an effect on the results. Certain conditions or illnesses might persist for just a specific amount of time (such as a common cold or the flu). There may also be a percentage of cases in a given population that will improve or completely recover from an illness spontaneously. Without a control condition, if researchers only looked at one group that received the test treatment, they may have attributed these recoveries to the intervention. However, with random assignment of subjects to a control condition, the overall results of the study should not be biased by this effect, as researchers should see a similar number of cases of spontaneous improvement in both study conditions.
Ethical Considerations: Standard of Care
The use of placebo conditions allows researchers to control for potential influences other than the intervention under study. This addresses the research’s scientific credibility. As previously stated, the ethical acceptability of a particular control group must also be weighed.
One ethical issue to consider is whether an effective therapy for the condition under study already exists. If one exists, the researchers must weigh the potential for harm to study subjects. In other words, the researchers must determine the potential harms of delaying or denying access to a treatment by assigning people to a placebo condition. The current standard of care represents the absolute minimum that people should receive in a treatment study. If a placebo treatment is beneath the current standard of care, it cannot ethically be administered.
Ethical Considerations: Rights, Safety, and Welfare
When designing a study, researchers must also consider any other issues affecting the rights, safety, and welfare of study subjects. Per the Office for Human Research Protections (OHRP), the Institutional Review Board (IRB) that oversees the research will make the determination as to whether the study design minimizes risks of harm to the study subjects (HHS 2018). The invitation to participate in research must also be extended in a non-coercive manner, and subjects must be fully informed with regard to existing therapies and the consequences of delaying treatment.
Ethical Considerations: Additional Information
In general, study subjects in control groups within preventive and therapeutic trials should receive an established effective intervention (in other words, the current standard of care). However, researchers may utilize placebos when there is no such established treatment. In addition, placebos may be used if withholding an effective intervention would not expose subjects to serious or irreversible harm.
Scientific and Ethical Hurdles
Depending on the intervention and topic of interest, researchers may encounter scientific and ethical hurdles in using the placebo-controlled design.
Scientific Hurdles
An example of a scientific hurdle is if researchers are examining a particular field of research where interventions already exist and represent the current standard of care. To illustrate, consider how many medications currently exist, both prescription and over the counter, to treat a mild headache. Taking medication for a headache would undoubtedly be better than taking an inactive substance. As a result, it would not be appropriate for researchers to use a placebo-controlled design unless they wanted to examine whether the intervention worked at all. If researchers want to examine how well an intervention works compared to others, the active comparator design is a better choice for an interventional study.
Ethical Hurdles
Ethically, it is inappropriate to utilize a placebo-controlled design if not offering the subjects in the study an intervention would subject them to undue harm; and an efficacious or effective intervention is available.
2. Active Comparator Design
Although an IRB can provide a specific determination as to whether the control condition is ethically appropriate, the active comparator design allows researchers to test how well the intervention works in a controlled study design while also ensuring that the control group is receiving a useful intervention. Within the active comparator design, researchers are essentially trying to see how well the chosen intervention (whether it is a new intervention or an existing standard) compares to other efficacious interventions. Information from such a study design could help to inform grant proposals or other funding applications by convincing others that the chosen intervention does something different than what already exists and is already available to the target population.
Active Comparator Design Challenges
Employing an active comparator study design can be statistically challenging. If the interventions in the treatment and control groups act via different mechanisms, researchers may essentially be comparing “apples to oranges” and not appropriately testing the efficacy of the chosen intervention. For example, if researchers were to compare an injectable method of pharmacological birth control versus male condoms in a study assessing risk of unintended pregnancy, they would essentially be comparing two methods of birth control that operate through different mechanisms. Moreover, the administration of the intervention would be supervised by a healthcare professional, whereas the control group would be utilizing an intervention that they would apply on their own. To more appropriately compare “apples to apples,” it would be advantageous for researchers to either compare the injectable method of birth control to another existing injectable birth control, or compare male condoms against female condoms, as their mechanisms would more closely overlap and allow the researchers to assess the individual efficacy of these interventions without the influence of potential confounders.
Design challenges can persist in ascertaining what intervention to utilize as the control group (Velentgas et al. 2012). This can be particularly challenging if there are multiple possibilities available to utilize, without clear evidence of which one may be more appropriate for the target population. By using available evidence and professional judgment, researchers may decide to utilize one particular existing intervention or a combination of available interventions. It is important to make this choice carefully, as it can affect the validity of study conclusions.
3. Cross-Over Design
When researchers utilize the cross-over design, they must assign study subjects to start out in one study condition and then after a period of time “cross over” to the other condition. Researchers can use this study design to compare two consecutively administered interventions or study an intervention using a placebo.
Cross-Over Design Using a Placebo
The following graphic illustrates a cross-over design using a placebo:
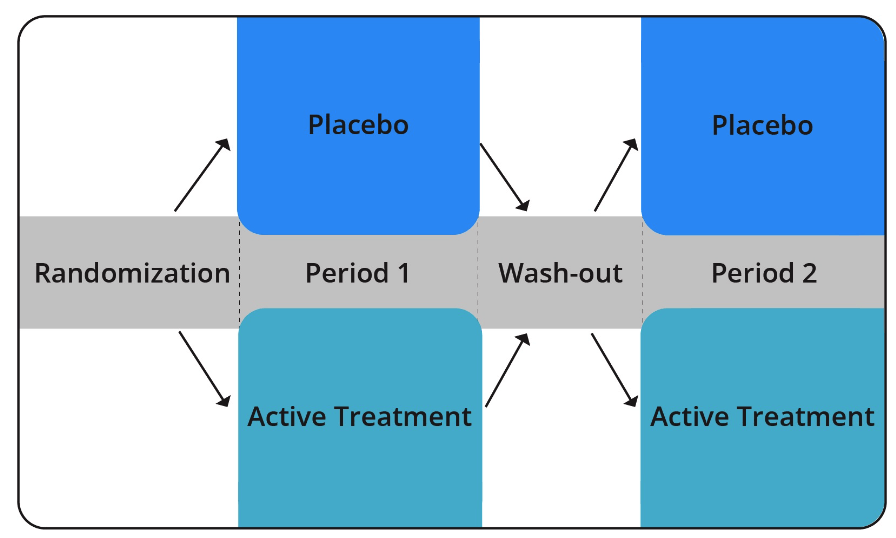
Subject assignment to a study condition is typically randomized such that half the subjects receive the intervention in one particular order (in other words, placebo during period 1 and active treatment in period 2), and half receive the intervention in the opposite order (in other words, active treatment in period 1 and placebo in period 2).
By utilizing this design, researchers ensure that each group receives both the placebo and active intervention at some point in the study. This allows them to compare each group’s experiences when they were receiving active treatment versus the placebo. Therefore, each group serves as its own control group.
Wash-out Period / Carry Over Effects
Before each group proceeds from period 1 to period 2, they go through what is called a “wash-out period.” The wash-out period is a time period that acts as a buffer, to ensure that any lingering effects of the first period’s intervention do not carry over to the second period’s intervention.
An example of a carry over effect is when a drug remains active in the body for a long period of time after an individual stops taking it. If the wash-out period was not long enough for the body to completely eliminate the drug from its system, some residual effects of the first period’s intervention could be attributed to the second period’s intervention.
4. Add-On Designs
When investigating treatments for certain diseases, it is medically and ethically inappropriate to consider the use of the placebo-control design. For example, researchers should not randomize subjects who suffer from congestive heart failure or cancer to a placebo. However, as noted with cross-over designs, other types of study designs, such as add-on designs, may still utilize a placebo as some component of the trial. Researchers often use add-on designs to study illnesses like heart failure, epilepsy, asthma, severe depression, and cancer when standard therapy is not totally effective.
Add-On Design Use
Consider a hypothetical example where researchers are investigating an investigational drug for congestive heart failure. If the researchers designed an active comparator trial, they would likely need a larger sample size in each condition to detect small differences in the effects of two different drugs. Although the placebo design would allow researchers to address the scientific issues, it would not be ethically appropriate to administer an inactive substance to subjects with life-threatening health conditions.
In this case, the add-on design would address the ethical issues of using placebo and avoid some of the methodological drawbacks of the active comparator design. The following graphic illustrates the add-on design:

In typical placebo trials, researchers restrict study subjects from other interventions. This is to avoid confounding factors and build greater confidence that the effect they are seeing is due to the intervention. However, an add-on design would allow study subjects to remain on their standard of care therapy. In other words, the researchers would still randomize subjects to the intervention or placebo, but those randomized to the placebo would still receive the standard of care treatment. This addresses the ethical problem presented by typical placebo trials.
Of course, when researchers make compromises to gain something, they must give up something in other areas. In this case, researchers could not be sure that the effects being seen were totally due to the investigational intervention. Results could be due to the standard of care treatment or an interaction between the investigational intervention and standard of care treatment. So, the results would only reflect the effect of the combination of the new intervention with the standard of care. Researchers still could not be certain that the new intervention would work as a stand-alone treatment.
Add-On Design Recap
Researchers can utilize the add-on design to compare an intervention to a placebo. The add-on design addresses some of the scientific issues presented by the active comparator design. Since subjects can remain on standard of care treatment, it also addresses some of the ethical issues presented by the placebo design. Add-on designs can also be useful when studying interventions for various clinical or behavioral outcomes.
Factorial Designs
In contrast to designs where the objective is to compare two different interventions, researchers can use a factorial design when the objective is to test different combinations of interventions.
5. Factorial Designs
In contrast to designs where the objective is to compare two different interventions, researchers can use a factorial design when the objective is to test different combinations of interventions.
Example
Using the cross-over design, researchers compare the effects of thiamine to placebo in controlling blood glucose levels. However, several factors may affect blood glucose levels.
Suppose researchers wish to compare the effects of thiamine in combination with other behaviors, which would more closely resemble real-world conditions. If the researchers want to test how thiamine works to control blood glucose when combined with a regimented exercise and/or nutrition program, they could consider employing a factorial design. In effect, subjects would be randomized twice: once to receive condition A or condition B, and then to receive condition C or condition D. So, the four groups would be A + C, A + D, B + C, and B + D. These four groups would be statistically compared to identify which combination of treatments would be most efficacious or effective.
6. Community Trial Use
Community trials are a type of interventional design where researchers manipulate an intervention variable and randomize entire groups to different arms of the study. Researchers often use these types of designs to assess the impact of policy decisions on public health. Similar to clinical trials, the first steps in a community trial include identifying an eligible community and gauging their willingness to participate. Government officials and/or community leaders usually give permission to enroll a particular community in a community trial.
Community trials typically assess interventions at the population level (such as changes in behavior that can prevent illness and injury). These studies may focus on issues such as smoking cessation, exercise, weight loss, and healthy nutrition.
Community-Based and High-Risk Approaches
Community trials favor a community-based approach over a high-risk approach (more on this later in the module). The community-based approach targets the entire population. This can be extremely efficient as the risk factors for many chronic diseases are usually common in a large percentage of the population. In addition, many cases of chronic disease occur in groups at low and intermediate risk. Therefore, even a small change in risk in a group will result in greater overall disease reductions than greater changes in a small group of high-risk individuals (Brownson et al. 1998). This phenomenon is known as the prevention paradox (Skog 1999).
In contrast, the high-risk approach targets individuals at the greatest risk. The identification of individuals with high blood glucose levels in the Diabetes Prevention Program is an example of this approach (DPP Research Group 2002b). The efficiency of the high-risk approach depends on the feasibility of identifying, correctly classifying, and changing the behavior of a high-risk group.
Example: Promoting Positive Change in Obesity-Related Risk Behaviors
A group of researchers are interested in childhood obesity and promoting positive change in obesity-related risk behaviors. The researchers should start with a literature review to gain some knowledge about their chosen area of study. Based on the literature review, the researchers find that parental obesity is associated with lower success rates in preventing childhood obesity (Nemet et al. 2008; Wrotnjak et al. 2004). This might prompt the researchers to target parents as part of the intervention.
Now, suppose the researchers are targeting two different schools for their study. They could randomly assign one school to receive the intervention and the other to receive no intervention. The intervention could be a counseling session on recommended ways to eat a nutritious diet and be physically active. The researchers could then decide to get some baseline data from the study subjects or start with some basic demographic data to describe the study population. Alternatively, they could choose additional information that is specifically relevant to the study (for instance, nutrition and exercise behaviors, including number of servings per day of fruits and vegetables, glasses of water consumed per day, hours per day spent engaging in physical activity, and hours per day spent watching television).
After a period of time, the researchers could then repeat the surveys of the two groups and evaluate whether the intervention was successful at improving parents’ obesity risk behaviors.
